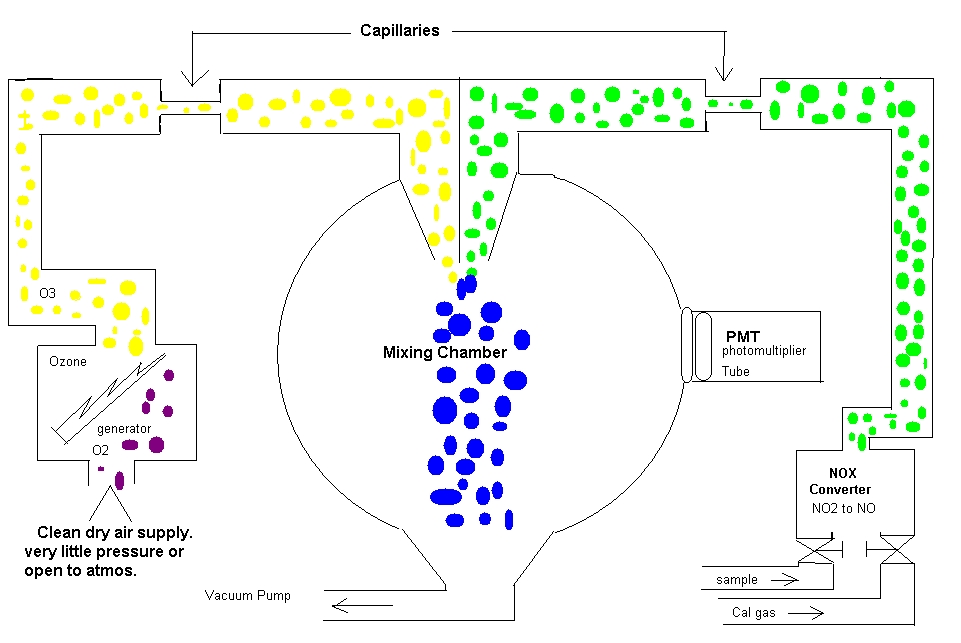
Chemiluminescence
Let me explain this in a semi technical fashion first and then in laymen terms. We are measuring for NO2. Nox has been determined to be one of the regulated components in our area and we must monitor for the state and try to reduce our emissions. Some common places where you will see this is on boilers and stacks. We will pull a sample down from the probe. As the sample comes into the analyzer it will go through a NOX converter and the NO2 will be converted to NO. After the sample is converted it will go through a flow restrictor (capillary) and then into the reaction chamber. We will feed in O3 to mix with the NO. To make O3 we will pull air through an ozone generator (ozonator) Inside the ozonator is a high voltage pulse continually firing. The O3 will go through a flow restrictor also. As these two meet they will produce a blue light. NO + O3 = NO2. This blue light is instantaneous and goes out very quickly. These lights are picked up on the photomultiplier tube (PMT). The signal is proportional to the amount of NO2.
In laymen terms. You are watching a fireworks show and you are in charge of counting the amount of fireworks that light up. You are the PMT. So they fire off a few and you count them, and then we do the finale, kinda like when we put the cal gas to it with a large amount of NOX. A huge display of blue light. Let’s say we run N2 into the analyzer. No fireworks, we are looking at zero.
Some important points about this analyzer. If you have a problem, make sure you are not out of cal gas. Check your flows and make sure the sample pump isn’t shot. If you have to open this up and work on it and you believe you have a flow issue. Pull the capillaries and clean them out. Be sure to mark them because they are not the same size for each side. Have a blue marker and a black one. Pull one and clean it and put it back and then pull the other after putting the one back in. If you don’t have a signal at all, pull and replace the ozonator. This is normally easy to do. We don’t want to power down the analyzer if we can help it because it is not healthy for the PMT. If this doesn’t help you call the 1-800- help. Have all of the information ready and they normally want you in front of the machine.
In laymen terms. You are watching a fireworks show and you are in charge of counting the amount of fireworks that light up. You are the PMT. So they fire off a few and you count them, and then we do the finale, kinda like when we put the cal gas to it with a large amount of NOX. A huge display of blue light. Let’s say we run N2 into the analyzer. No fireworks, we are looking at zero.
Some important points about this analyzer. If you have a problem, make sure you are not out of cal gas. Check your flows and make sure the sample pump isn’t shot. If you have to open this up and work on it and you believe you have a flow issue. Pull the capillaries and clean them out. Be sure to mark them because they are not the same size for each side. Have a blue marker and a black one. Pull one and clean it and put it back and then pull the other after putting the one back in. If you don’t have a signal at all, pull and replace the ozonator. This is normally easy to do. We don’t want to power down the analyzer if we can help it because it is not healthy for the PMT. If this doesn’t help you call the 1-800- help. Have all of the information ready and they normally want you in front of the machine.
Let's take a look at one of the ways to control Nox emissions today.
Selective Catalytic Reduction
Selective Catalytic Reduction
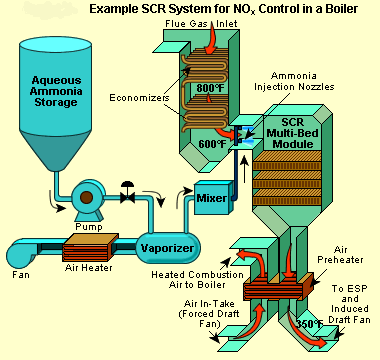
Selective catalytic reduction (SCR) is a means of converting nitrogen oxides, also referred to as NOx with the aid of a catalyst into diatomic nitrogen, N2, and water, H2O. A gaseous reductant, typically anhydrous ammonia, aqueous ammonia or urea, is added to a stream of flue or exhaust gas and is absorbed[citation needed] onto a catalyst. Carbon dioxide, CO2 is a reaction product when urea is used as the reductant.
Selective catalytic reduction of NOx using ammonia as the reducing agent was patented in the United States by the Englehard Corporation in 1957. Development of SCR technology continued in Japan and the US in the early 1960s with research focusing on less expensive and more durable catalyst agents. The first large-scale SCR was installed by the IHI Corporation in 1978.[1]
Commercial selective catalytic reduction systems are typically found on large utility boilers, industrial boilers, and municipal solid waste boilers and have been shown to reduce NOx by 70-95%.[1] More recent applications include diesel engines, such as those found on large ships, diesel locomotives, gas turbines, and even automobiles. including cars
Selective catalytic reduction of NOx using ammonia as the reducing agent was patented in the United States by the Englehard Corporation in 1957. Development of SCR technology continued in Japan and the US in the early 1960s with research focusing on less expensive and more durable catalyst agents. The first large-scale SCR was installed by the IHI Corporation in 1978.[1]
Commercial selective catalytic reduction systems are typically found on large utility boilers, industrial boilers, and municipal solid waste boilers and have been shown to reduce NOx by 70-95%.[1] More recent applications include diesel engines, such as those found on large ships, diesel locomotives, gas turbines, and even automobiles. including cars
_______________________________