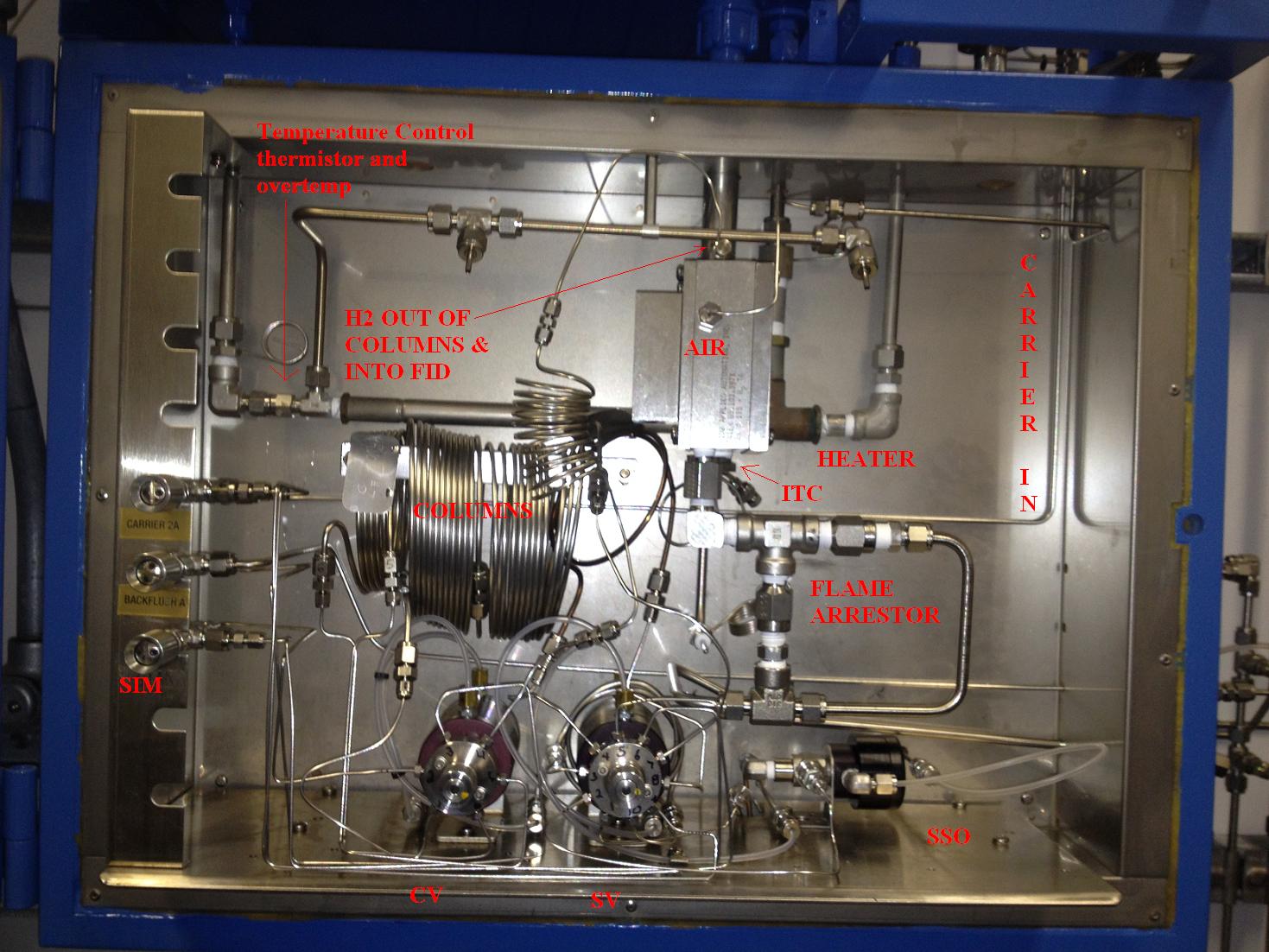
Flame Ionization Detector
It is very rare that you will have to change out an FID, I've had to do it twice now, lucky damn me. In order to have a flame you have to have three things Air, Fuel, & a Spark. In this application we are using H2 as the carrier and the fuel. This is an Advance Optichrom that has been revamped to be an HRVOC analyzer looking for C2= and C3=.
It's a very easy to do application but they complicated it by making it a trap bypass. The ethylene comes out of column two and goes into the CV which sends it to column 3. Then the valve turns off thus trapping C2= there while we let the propylene go through the column simulator and to the FID. Then we release the trap and let ethylene go to the detector.
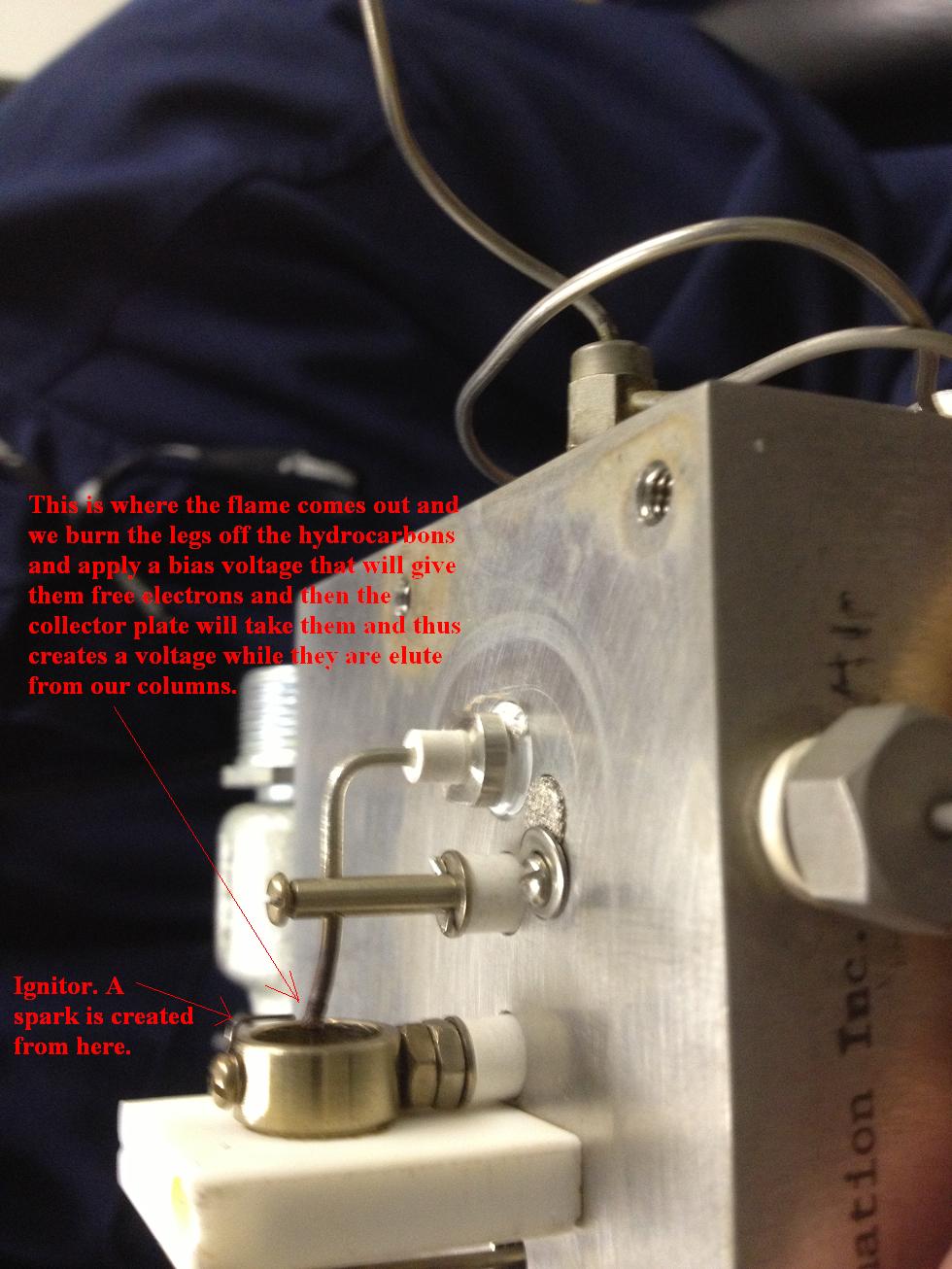
You know that you must have fire to make an FID work. You will have to have fuel, this is going to be H2. Some times they use H2 as the carrier and fuel. Not all the time though. Remember that an FID can only see hydrocarbons and only in small amounts like ppm. If you send a large amount of hydrocarbons to the FID it will saturate it. Like tossing a barrel of gas on a campfire. We will inject a sample and separate the hydrocarbons with our columns and as they elute out and are carried to the detector. They will pass through the flame. As the hydrocarbons pass through the flame it will burn their hydrogen's off. Each carbon has four legs and had 4 hydrogen's before they burned off thus now leaving our carbon hunting for electrons. We will hand it four electrons from a bias voltage right at the flame but then we count those electrons on a collector plate. So we are giving electron and taking them away and counting them. It literally creates a current flow and this is what the detectors shows in the chromatogram.
It can only see hydrocarbons. It cannot see N2, O2, and so on. If the FID is showing a baseline then there is nobody burning up.
_________________________________